Neonatal Fetal Nutrition & Metabolism
Neonatal Fetal Nutrition & Metabolism 1: Neonatal and Fetal Metabolism
299 - Hypoinsulinemic Hyperglycemia Worsens the Adverse Effects of Perinatal Iron Deficiency on the Hippocampus in Rats
Publication Number: 299.128
- KE
Kathleen M. Ennis-Czerniak
Researcher
University of Minnesota
minneapolis, Minnesota, United States
Presenting Author(s)
Background:
Perinatal iron deficiency (ID) and hypoinsulinemic hyperglycemia (HG) are co-morbidities in extremely preterm infants (EPT). Hippocampus (HPC)-based long-term cognitive deficits are common in these infants. Both ID and HG target the HPC in animal models. Whether HG worsens the adverse effects of ID on the HPC is not known.
Objective: Examine whether HG worsens the acute and long-term adverse effects of ID on the HPC in a rat model.
Design/Methods: Pregnant rat dams were fed an iron sufficient (IS, 200 ppm) or ID (3-6 ppm) diet from gestational day 3 through postnatal day (P) 7. Hypoinsulinemic HG was induced by injecting streptozotocin (STZ 60mg/kg) on P2 (neurodevelopment equivalent to human EPT). On P7, mRNA expression of iron transporters, transferrin receptor (Tfrc) and divalent metal transporter-1 (Dmt1) and marker of oxidative stress, poly(ADP-ribose) polymerase 1 (Parp1) in the HPC were determined using qPCR. On P65, microglia in HPC subareas were determined using histochemistry (N = 6/group).
Results: Hematocrit (Hct, %) was lower in the ID and ID+STZ groups (IS: 33.2 ± 0.94, IS+STZ: 35.3 ± 0.65, ID: 13.9 ± 0.48, ID+STZ: 20.3 ± 0.83, p< 0.001; Fig 1A) on P7 and had normalized on P65 (Fig 1B). On P7, blood glucose (mg/dL) was elevated in the STZ groups, but did not differ between the IS and ID groups (IS: 115.0 ± 3.3, ID: 95.0 ± 3.9, IS+STZ: 226.8 ± 12.1, ID+STZ: 189.4 ± 15.7, p< 0.001). Relative to the IS group, Tfrc (+56%) and Dmt1 (+62%) expression was upregulated in the ID group (p<0.01). HG led to further Tfrc (243%) upregulation in the ID+STZ group (p< 0.0001; Fig 2A) and Dmt1 upregulation in the IS+STZ (38%) and ID+STZ (96%) groups (p=0.01; Fig 2B). Parp1 expression was increased (154%) only in the ID+STZ group (p< 0.0001 Fig 2C). On P65, there were more microglia in HPC CA1 subarea in the ID+STZ group compared with the IS, ID and IS+STZ groups (Fig 3).
Conclusion(s): Increased Tfrc and Dmt1 expression indicate HPC tissue ID. Drastic upregulation of Tfrc and Dmt1 in the ID+STZ group suggests that HG worsens HPC ID. Parp1 upregulation in ID+STZ group suggests that HG causes oxidative stress in the ID HPC. Increased number of microglia in CA1 in the ID+STZ group could be a long-term sequelae of HG-induced oxidative stress and suggest that HG worsens impaired HPC development in perinatal ID.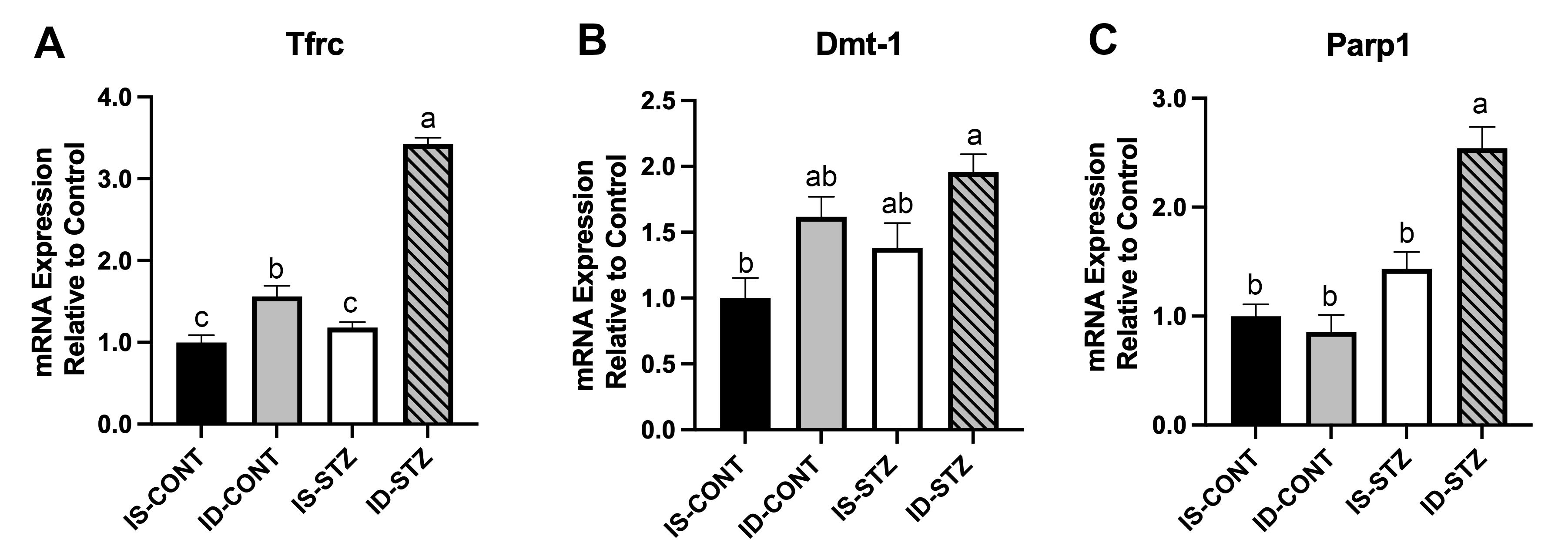
.jpg)
.jpg)
