Neonatal Quality Improvement
Neonatal Quality Improvement 1
651 - Quality Improvement Initiative to Decrease Antibiotic Utilization in Low-Risk Preterm Infants < 35 Weeks in a Level IV Neonatal Intensive Care Unit
Friday, April 28, 2023
5:15 PM - 7:15 PM ET
Poster Number: 651
Publication Number: 651.138
Publication Number: 651.138
Giselle Gozum, NewYork-Presbyterian Komansky Children’s Hospital, New York, NY, United States; Priyanka Tiwari, Weill Cornell Medicine, New Hyde Park, NY, United States; Lauren Blatt, Weill Cornell Medicine, New York, NY, United States; Vivien Yap, Weill Cornell Medicine, New York, NY, United States; Liana Senaldi, Weill Cornell Medicine, New York, NY, United States

Giselle Gozum, MD (she/her/hers)
Clinical Fellow
NewYork-Presbyterian Komansky Children’s Hospital
New York, New York, United States
Presenting Author(s)
Background: Many preterm infants receive a course of antibiotics for concern for early-onset sepsis (EOS). A clinical report from the American Academy of Pediatrics in 2018 suggests potential risk stratification strategies for limiting initiation of antibiotics in this population by considering the balance between the circumstance of preterm birth and the clinical condition of the preterm infant.
Objective: To reduce initiation of antibiotics in low-risk preterm infants born between 30 to 34 6/7 weeks gestation by 50% and to reduce total antimicrobial use in a level IV neonatal intensive care unit (NICU) by 10% by January 2023.
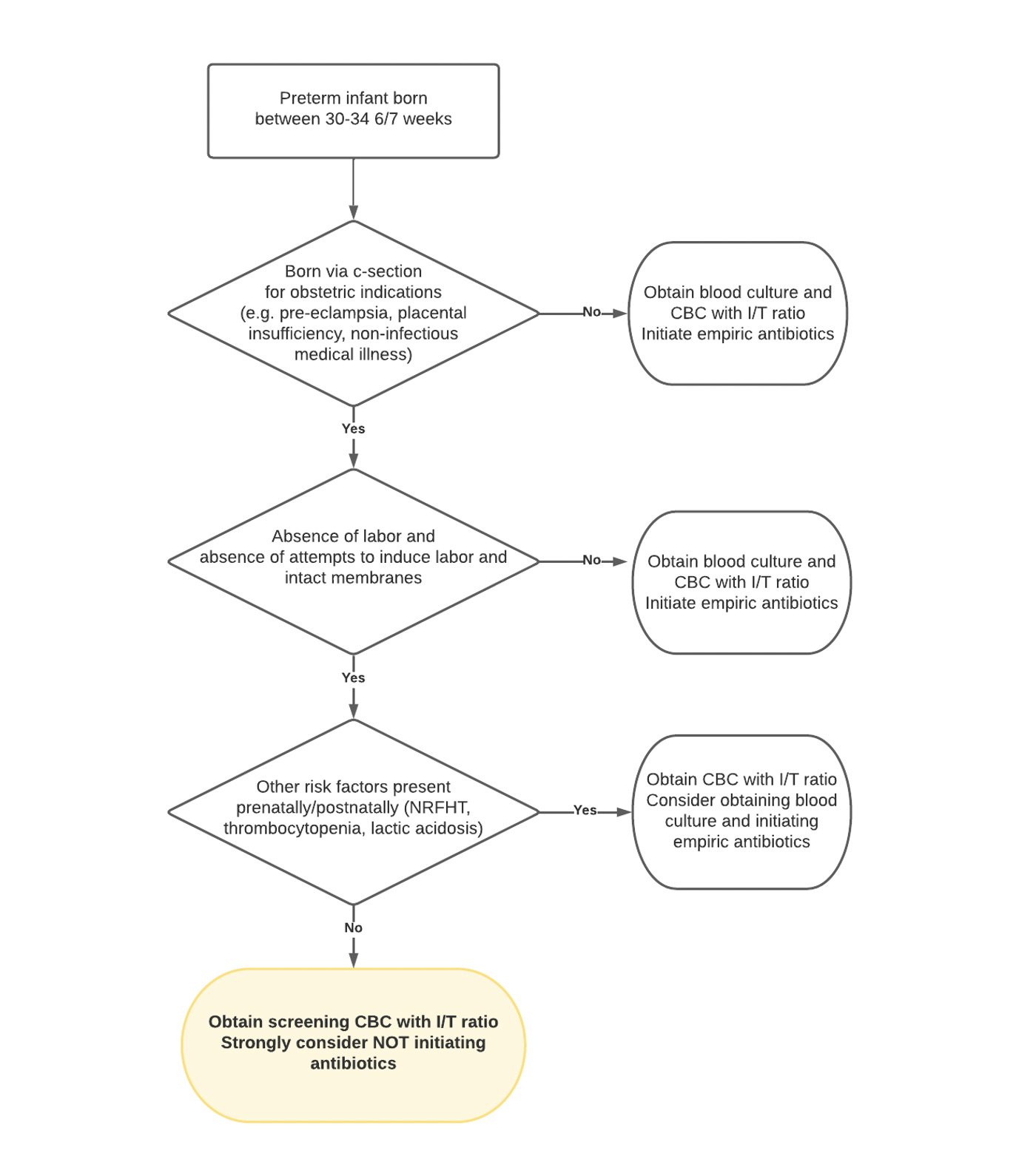
Design/Methods: A multidisciplinary NICU antimicrobial stewardship program (ASP) was created in 2019, with a new initiative launched in 2021 to limit antibiotic use for low-risk preterm infants 30 to 34 6/7 weeks gestation admitted to NewYork-Presbyterian-Weill Cornell NICU. These infants were born via cesarean section for maternal indication without labor or rupture of membranes, with no other risk factors for EOS (Figure 1). Process and outcome measures included percentage of target population initiated on antibiotics, total antibiotic days of therapy (DOT)/1000 patient days, and EOS-specific DOT/1000 patient days. Balance measures included blood cultures and clinical outcomes. Statistical process control charts were used to display and analyze data. Associates for Process Improvement rules for special cause were applied.
Results: There was a decrease in initiation of antibiotics in low-risk preterm infants from 100% to 33% following the establishment of a NICU ASP and the creation of the preterm clinical pathway, and further down to 6% (n=129, Figure 2). There was a 27% reduction in the total NICU antibiotic DOT/1000 patient days (Figure 3) since the initiation of an ASP and subsequent interventions. Special cause variation was noted in the EOS antibiotics of ampicillin and gentamicin, resulting in 23% and 28% reduction in the DOT/1000 patient days, respectively. No cases of EOS (suspected or culture-positive) occurred in preterm infants who did not receive antibiotics. Infants in our target population who were started on antibiotics were more likely to require intubation and/or have a birth weight < 1500 g (P< 0.05).
Conclusion(s): An intervention to limit initiation of antibiotics in low-risk infants born between 30 to 34 6/7 weeks gestation was successful in reducing antibiotic exposure and NICU antimicrobial use and was not associated with adverse outcomes. The most impactful interventions were creation of a NICU ASP and the creation of a clinical pathway for low-risk preterm infants.
.png)
.png)
