Pharmacy and Therapeutics: Medication Quality Improvement
Pediatric Therapeutics and Pharmacology
778 - Physiologically Based Pharmacokinetic Approach to Inform Cefepime Dosing in Children on Extracorporeal Membrane Oxygenation
Publication Number: 778.148

Danielle J. Green, MD
Visiting Instructor of Pediatrics
University of Utah School of Medicine
Salt Lake City, Utah, United States
Presenting Author(s)
Background: Extracorporeal membrane oxygenation (ECMO) is a life-saving technology in children with refractory cardiac and/or respiratory failure. Children supported with ECMO often receive cefepime to treat confirmed or suspected infections. Unfortunately, cefepime lacks dosing information specific to children supported with ECMO. Drug dosing is different in this population because the ECMO circuit components and physiologic alterations triggered by critical illness, such as hypoalbuminemia and renal dysfunction, substantially alter drug pharmacokinetics (PK). These PK alterations place patients at risk for treatment failure and toxicity.
Objective: Develop a cefepime physiologically based pharmacokinetic (PBPK) model to translate results from ex vivo drug-circuit interaction studies into bedside dosing recommendations in children on ECMO.
Design/Methods: We first developed a full-body, pediatric PBPK model using physiochemical properties of cefepime and PK data in adults and children from the literature. Then, an ECMO compartment was parameterized using data from our previously published ex vivo study and added to the PBPK model. The ECMO PBPK model was then adapted to include hypoalbuminemia and renal dysfunction that are common in children supported with ECMO. ECMO PBPK model predictions were validated using in vivo cefepime concentration data collected from children on ECMO. Predictions were evaluated by comparing in vivo plasma cefepime concentrations to the 90% prediction intervals.
Results:
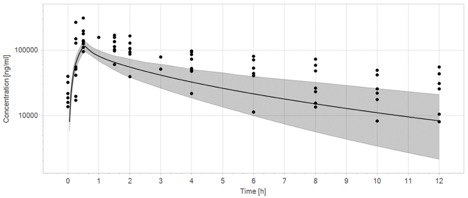
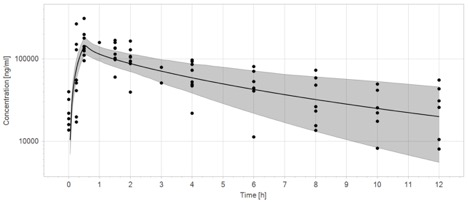
The initial ECMO PBPK model captures only 40% (23/57) of the observed data within the 90% prediction interval (Figure 1a). Plasma concentrations are underpredicted in both the distribution and elimination phases. In contrast, after incorporating hypoalbuminemia and renal dysfunction, the final ECMO PBPK model captures 68% (39/57) of the observed data within the 90% prediction interval (Figure 1b).
Conclusion(s):
The PK of cefepime are altered in children supported with ECMO. Compared with children not supported with ECMO, children on ECMO have increased volume of distribution and decreased clearance of cefepime. This work demonstrates that ex vivo experiments may be combined with pediatric PBPK models which incorporate critical illness to predict cefepime disposition in this complex and vulnerable population. Next steps in this work include developing cefepime ECMO dosing recommendations for children across the age spectrum.