Pharmacy and Therapeutics: Medication Safety Research
Pediatric Therapeutics and Pharmacology
779 - Prevalence of Prescription of Drugs with a High Level of Pharmacogenetic Evidence in Children with Medicaid Insurance
Publication Number: 779.148

Sonya C. Tang Girdwood, MD, PhD (she/her/hers)
Assistant Professor of Pediatrics
Cincinnati Children' Hospital Medical Center
Cincinnati, Ohio, United States
Presenting Author(s)
Background:
The number of drugs with a high level of evidence for pharmacogenetic-guided dosing (PGx drugs) in children is rapidly increasing, but guidance on implementation of pharmacogenetic (PGx) testing, including in which pediatric populations, is limited. How best to implement PGx testing could be informed by knowing which PGx drugs are most frequently prescribed, the genes that affect these frequently prescribed PGx drugs, and the characteristics of the patients who are prescribed PGx drugs.
Objective:
To determine the prevalence of PGx drug prescriptions and to describe the characteristics of children who are prescribed PGx drugs.
Design/Methods:
We performed serial cross-sectional analyses of PGx drug prescribing in children <18 years using the Truven Medicaid database from 2011-2019. We defined PGx drugs as those having Level A PGx evidence from the Clinical Pharmacogenetics Implementation Consortium in current or upcoming guidelines. We determined the prevalence of PGx drug prescriptions in all years. Drugs were then grouped by the associated Level A gene(s) to determine the prescribing rate per 100,000 children in 2019. Characteristics of children prescribed at least 1 PGx drug were compared to children not prescribed PGx drugs in 2019.
Results:
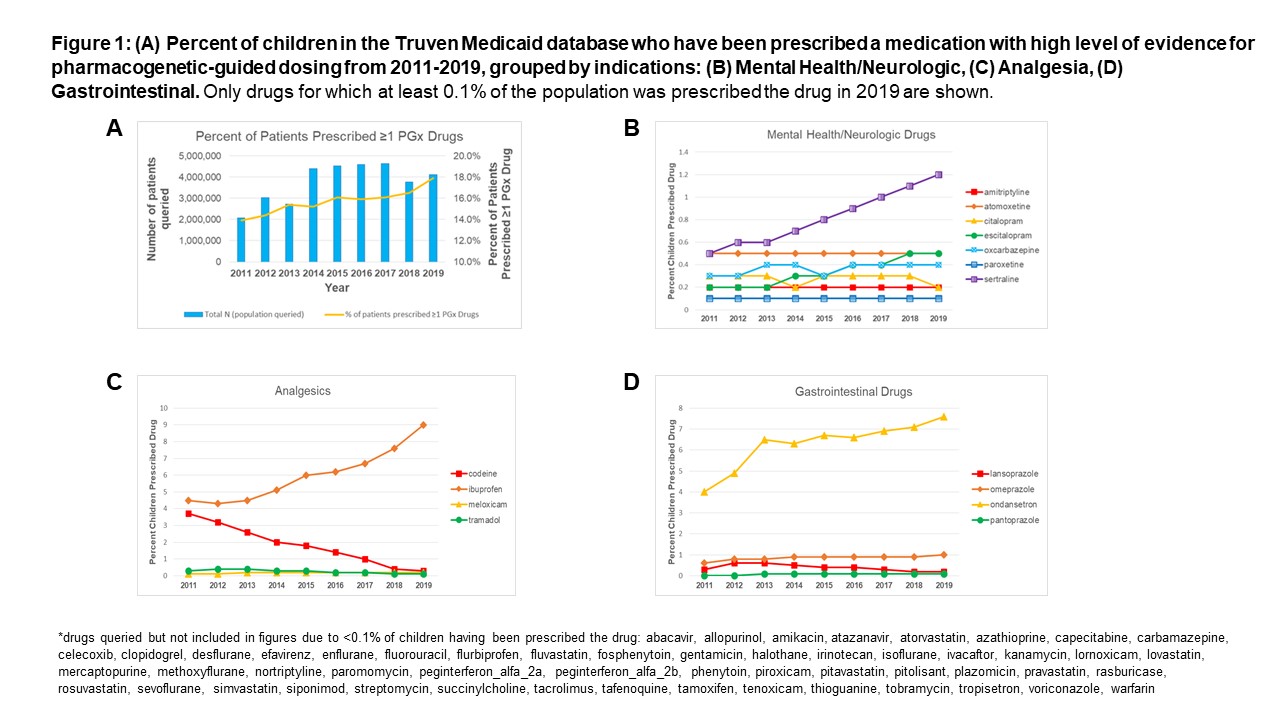
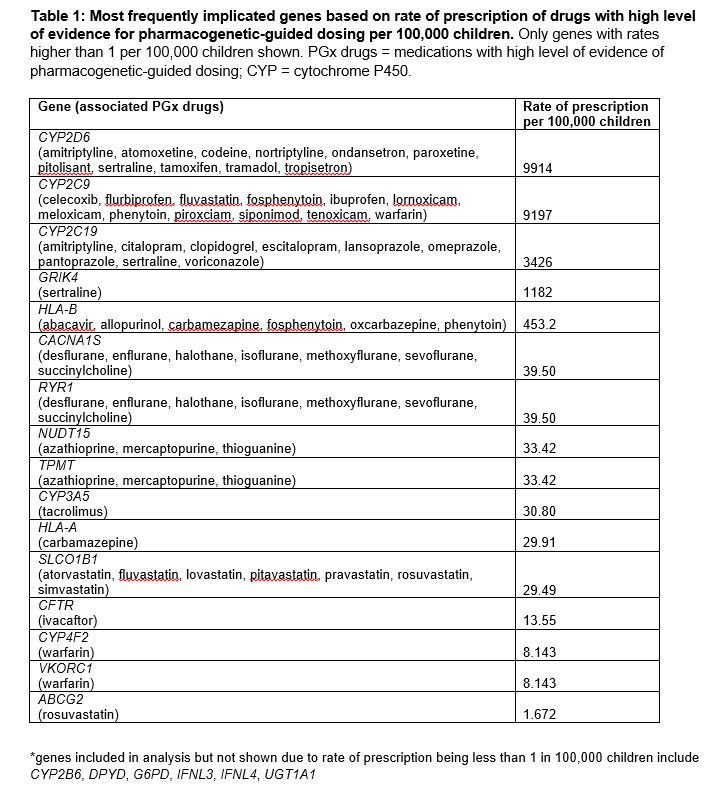
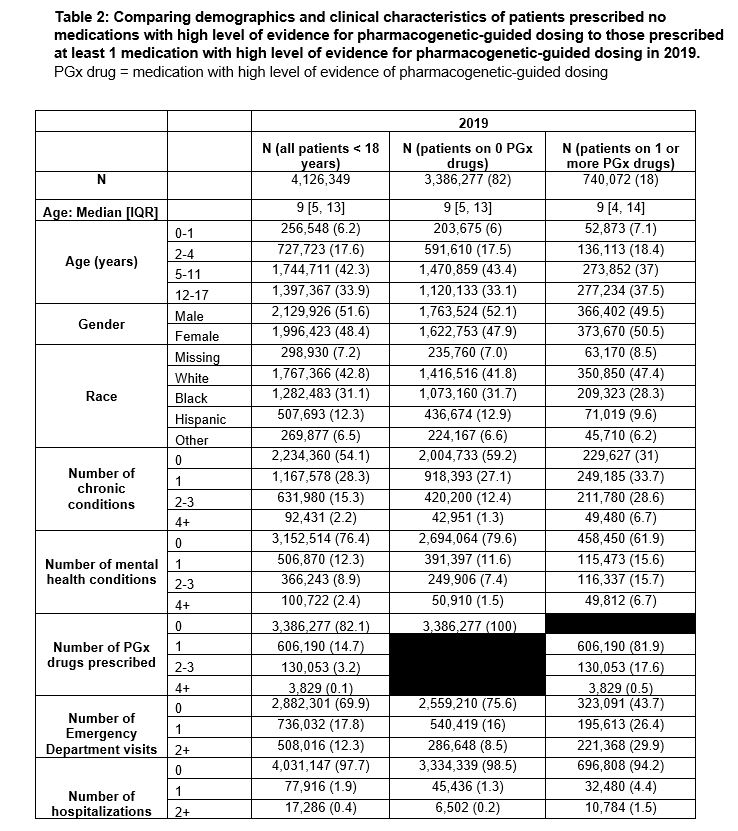
Between 2011 and 2019, the percentage of children prescribed ≥1 PGx drugs increased from 13.9% to 17.9% (Figure 1A). PGx drugs prescribed to ≥0.5% of the Medicaid-insured population in 2019 include ibuprofen (9%), ondansetron (7.6%), sertraline (1.2%), omeprazole (1%), atomoxetine (0.5%) and escitalopram (0.5%) (Figure 1B-D). Codeine was prescribed to 3.7% of children in 2011 but only to 0.3% of children in 2019 (Figure 1C). Genes associated with PGx drugs with the highest prescribing rates in 2019 were CYP2D6 (9913 per 100,000), CYP2C9 (9197 per 100,000), and CYP2C19 (3426 per 100,000) (Table 1). Compared to those not prescribed PGx drugs, children prescribed ≥1 PGx drug were more likely to have ≥1 chronic medical or mental health condition(s), more emergency room visits, and more hospitalizations (Table 2).
Conclusion(s):
Prescribing of drugs with a high level of PGx evidence has increased over the last decade, indicating increasing opportunities for PGx-guided dosing to improve safe medication use in children. PGx testing should initially focus on the most influential genes associated with highly prescribed drugs, particularly for higher risk children with chronic medical or mental health conditions.