Neonatal Quality Improvement
Neonatal Quality Improvement 4
746 - Premature Transfusions of Premature Infants- A Quality Improvement Study
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 746
Publication Number: 746.343
Publication Number: 746.343
Charlene R. Bultmann, University of Virginia School of Medicine, Charlottesville, VA, United States; Brynne Sullivan, University of Virginia, Charlottesville, VA, United States; Jonathan R. Swanson, University of Virginia School of Medicine, Charlottesville, VA, United States
.jpg)
Charlene R. Bultmann, DO (she/her/hers)
Neonatal-Perinatal Medicine Fellow
University of Virginia School of Medicine
Charlottesville, Virginia, United States
Presenting Author(s)
Background: Preterm infants are commonly transfused at higher hemoglobin levels. Recent randomized clinical trials suggest that increasing the threshold for transfusion of packed red blood cells (PRBCs) has no negative effects on neurodevelopmental outcomes.
Objective: To decrease transfusions of PRBCs in preterm infants outside of unit protocol.
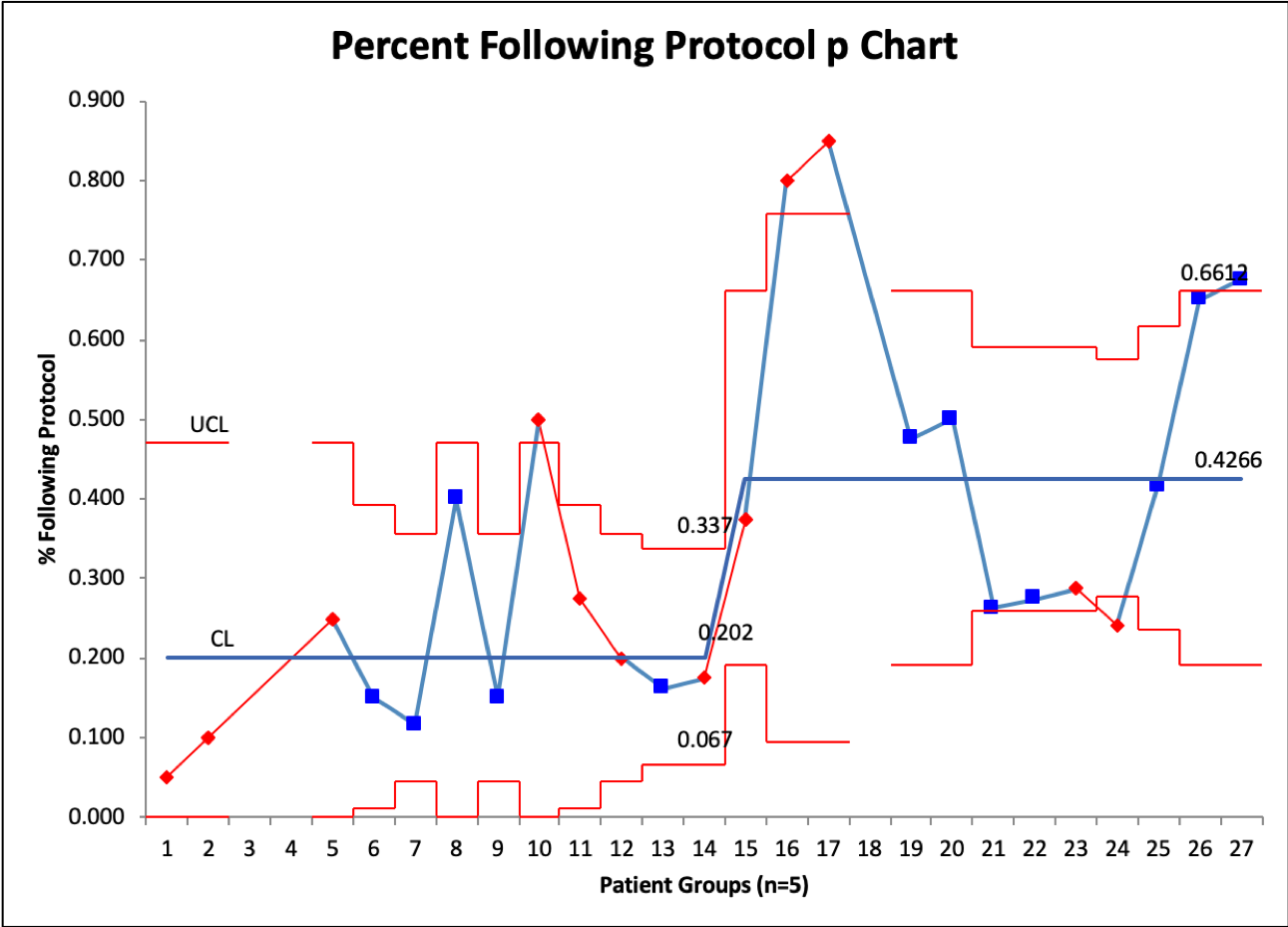
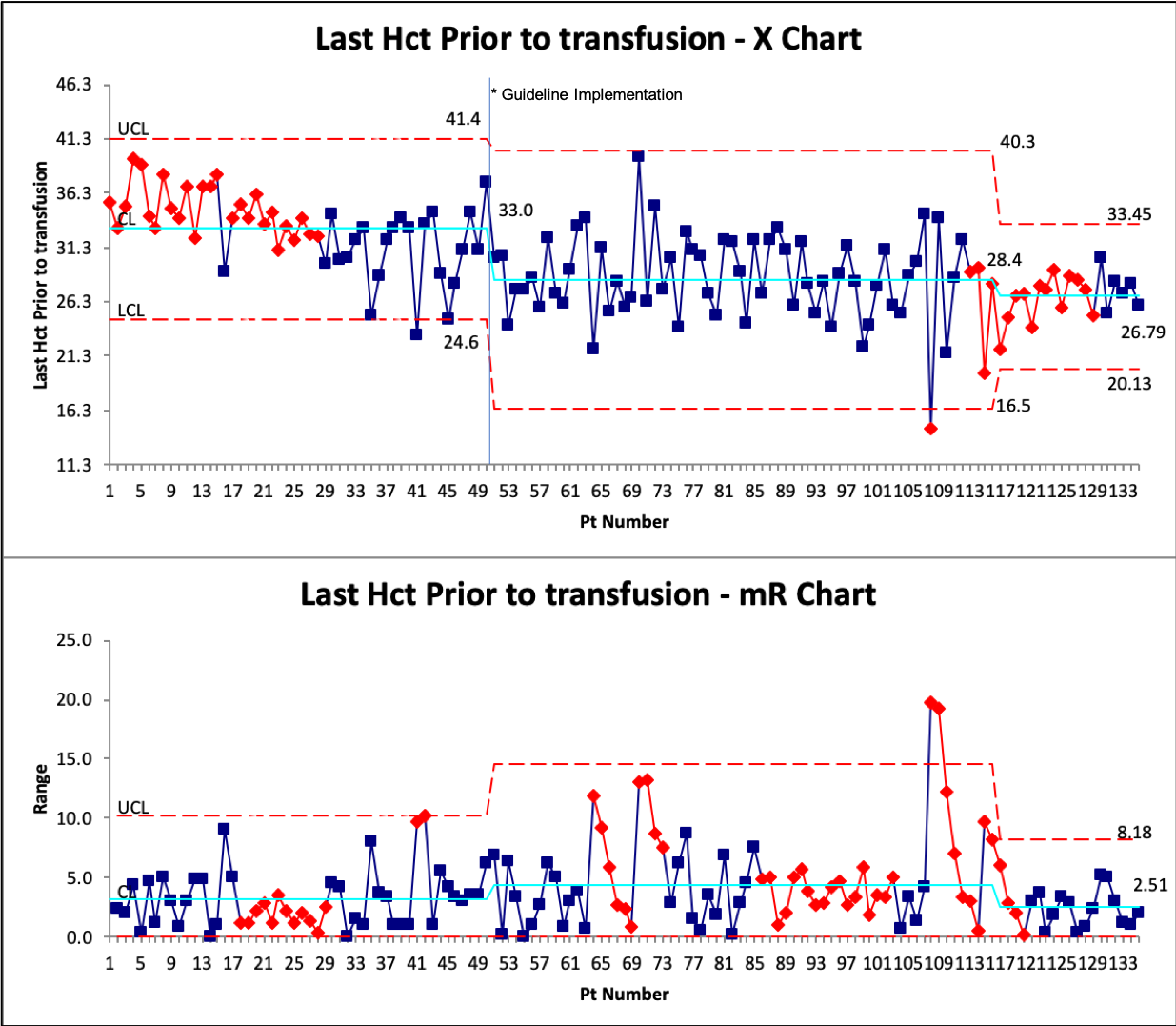
Design/Methods: In May 2022, the University of Virginia NICU reviewed recent clinical trials regarding PRBC transfusions in all premature infants less than 37 weeks. A new transfusion protocol was developed and implemented in July 2022. Journal clubs, direct education to ordering providers, and unit-wide education was provided on the new protocol. Baseline data was collected between April-June 2022 on last hematocrit prior to transfusion and if the transfusion followed unit protocol. Transfusion data were followed prospectively from July to November. Process control charts were used to monitor the intervention’s outcome. P-chart was used to monitor guideline adherence (using groups of 5 patients) and an X-bar R chart was used to evaluate the last hematocrit prior to transfusion. Balancing measures of necrotizing enterocolitis and length of stay were also monitored.
Results: A total of 51 transfusions were included in pre-implementation and 86 post implementation. The average gestational age was 25.6 weeks. X bar R chart demonstrated a change in in last hematocrit prior to transfusion from 33.0% to 28.0% after the intervention and then to 26.8%. However, there was an increase in variability as demonstrated by the R chart with several transfusions outside the control limits. Adherence to the protocol improved from a median of 20% of patients to just over 40% after intervention. Several special cause variations were noted.
Conclusion(s): Implementation of an evidenced-based protocol for PRBC transfusions improved protocol adherence and reduced the average hematocrit prior to transfusion. However, there remains significant variation which suggests further efforts to improve adherence are needed.