Back
Background: Improvement in neonatal cardiac surgery mortality and morbidity remains an ongoing challenge. Known risk factors do not fully capture differences in outcomes in children undergoing cardiac surgery. Metabolomics enables capture of the complex interplay between genetic and environmental variables, and its use has been applied in various clinical and non-clinical contexts to predict phenotypic expression. Thus far, data is limited in pediatric populations.
Objective: Identify metabotypes and how these relate to outcomes in neonates undergoing cardiac surgery.
Design/Methods: This is a secondary study of patient characteristics and outcomes from the Methylprednisolone Trial. Targeted serum metabolomics were performed with LC/mass spectroscopy from perioperative samples collected prior to skin incision, immediately post-operatively, and 12 hours post- operatively. Patient characteristics, outcomes, metabolite data and their derived features were included in principal component analysis. Selected principal components (PCs) were then used for unsupervised k- means clustering analysis to identify potential clusters of patients with similar metabolic profiles. Differences in patient characteristics and clinical status between clusters were assessed using Fisher’s exact test, or linear regression and Wald chi-square statistics.
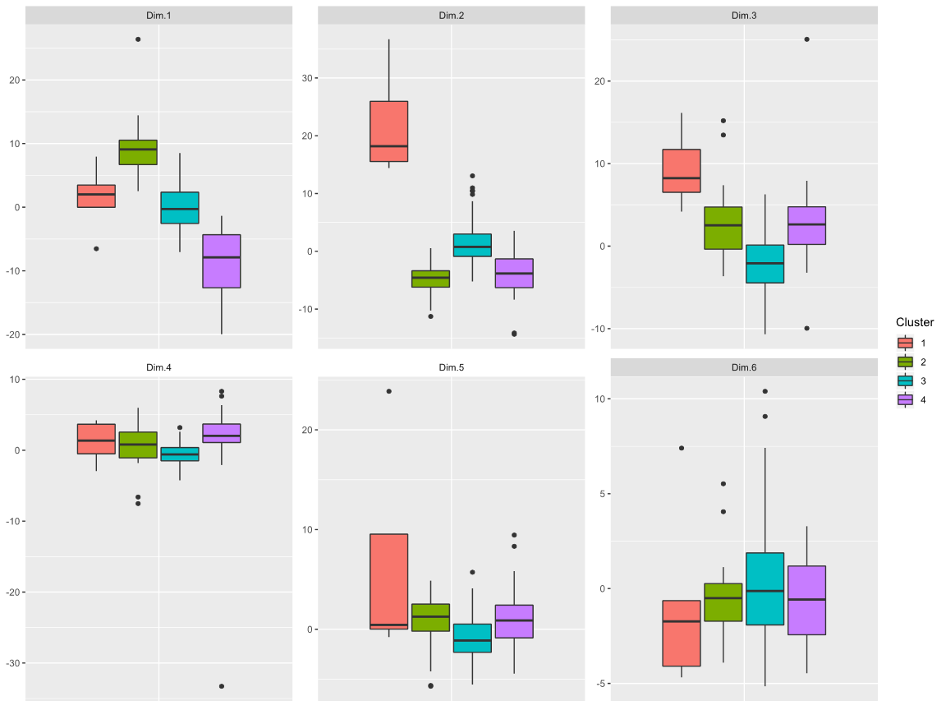
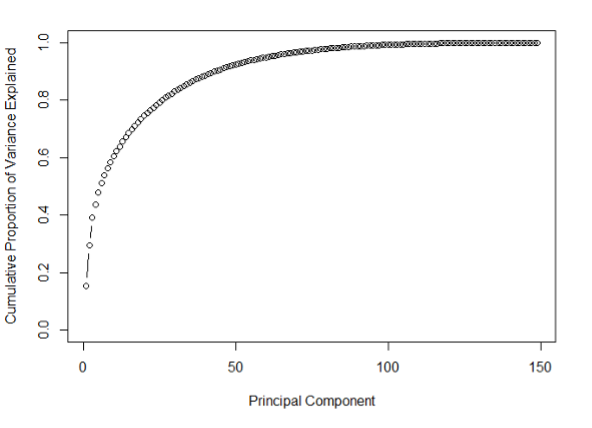
Results: From a total cohort of 149 patients, 40 serum metabolites were quantifiable and resulted in 6 PCs that explained 51% of cumulative variance. The 6 metabolite PCs identified 4 distinct metabotype clinical clusters labeled A-D. Cluster B had worse outcomes overall, such as lowest urine output post- operatively, longest length of mechanical ventilation, liver injury (58%), and death (15%), whereas cluster D had the best outcomes including lowest rates of post-operative complications. Operative variables such as heart disease diagnosis, bypass or crossclamp time, and Society of Thoracic Surgery (STS) risk score were not different between clusters. Only the PC1 metabolite profile, which was mostly pre-operative, was increased in the poor outcome (cluster B) and decreased in good outcome (cluster D) clusters.
Conclusion(s): Unsupervised clustering identified groups of patient characteristics and distinguished groups with better or worse outcomes according to a common metabolic profile. Metabotypes, composed predominantly of pre-operative metabolite values, may be possible to predict adverse outcomes and provide a readout to identify pre-operative clinical variables that drive outcomes.
.png)
Cardiology
Cardiology 2
757 - Perioperative Metabotypes Associated with Post-operative Outcomes in Neonates Undergoing Cardiopulmonary Bypass
Sunday, April 30, 2023
3:30 PM – 6:00 PM ET
Poster Number: 757
Publication Number: 757.304
Publication Number: 757.304
Rebecca Dryer, Johns Hopkins - - City not selected, MD, Baltimore, MD, United States; Jessica Heibel, Pediatrix Cardiology of San Antonio, San antonio, TX, United States; Allen D. Everett, Johns Hopkins University School of Medic, Glenwood, MD, United States; Cedric Manlhiot, Johns Hopkins Children's Center, Baltimore, MD, United States; Aurelie ROUX, Johns Hopkins University School of Medicine, Saint Petersburg, FL, United States; David Graham, Johns Hopkins University School of Medicine, Germantown, MD, United States; Eric Graham, Medical University of South Carolina, Charleston, SC, United States

Rebecca Dryer, MD (she/her/hers)
Resident Physician
Johns Hopkins - - Baltimore, MD
Baltimore, Maryland, United States
Presenting Author(s)
Background: Improvement in neonatal cardiac surgery mortality and morbidity remains an ongoing challenge. Known risk factors do not fully capture differences in outcomes in children undergoing cardiac surgery. Metabolomics enables capture of the complex interplay between genetic and environmental variables, and its use has been applied in various clinical and non-clinical contexts to predict phenotypic expression. Thus far, data is limited in pediatric populations.
Objective: Identify metabotypes and how these relate to outcomes in neonates undergoing cardiac surgery.
Design/Methods: This is a secondary study of patient characteristics and outcomes from the Methylprednisolone Trial. Targeted serum metabolomics were performed with LC/mass spectroscopy from perioperative samples collected prior to skin incision, immediately post-operatively, and 12 hours post-
Results: From a total cohort of 149 patients, 40 serum metabolites were quantifiable and resulted in 6 PCs that explained 51% of cumulative variance. The 6 metabolite PCs identified 4 distinct metabotype clinical clusters labeled A-D. Cluster B had worse outcomes overall, such as lowest urine output post-
Conclusion(s): Unsupervised clustering identified groups of patient characteristics and distinguished groups with better or worse outcomes according to a common metabolic profile. Metabotypes, composed predominantly of pre-operative metabolite values, may be possible to predict adverse outcomes and provide a readout to identify pre-operative clinical variables that drive outcomes.


.png)
