Hospital Medicine: Clinical
Hospital Medicine 6
759 - Misclassification of influenza infection and oseltamivir exposure status in administrative data
Publication Number: 759.415

Hannah K. Bassett, MD (she/her/hers)
Clinical Assistant Professor
Stanford University School of Medicine
Palo Alto, California, United States
Presenting Author(s)
Background: The AAP statement on influenza recommends that patients hospitalized with suspected or confirmed influenza be treated with a neuraminidase inhibitor (NAI) regardless of symptom duration. Prior studies using administrative data have demonstrated an association between NAI use and improved clinical outcomes. However, administrative data may not account for the clinical context around treatment decisions or may misclassify patients based on diagnosis codes, leading to potential bias in results.
Objective:
We characterized reasons for apparent non-adherence to AAP influenza guidelines in children hospitalized with ICD-9 and -10 codes for influenza. We also compared clinical outcomes in accurately vs inaccurately classified patients.
Design/Methods: Retrospective cohort study conducted at a children’s hospital that participates in the Pediatric Health Information System (PHIS) database. We mirrored the approach by Walsh et al (JAMA Pediatr 2022) to identify patients in PHIS at our hospital: patients < 18 years; discharged between 1/2014 and 3/2020 with a 1° or 2° discharge ICD-9 or -10 code for influenza; no billing code for oseltamivir; and length-of-stay (LOS) ≥ 2 days. Using chart review, we categorized patients as being accurately or inaccurately classified as patients with active influenza who did not receive oseltamivir during hospitalization but should have according to AAP guidelines. We compared LOS and in-hospital ECMO and/or death (outcomes used by Walsh et al) between these two cohorts.
Results: For the 87 patients meeting our inclusion criteria, median (IQR) age was 4 (2-8) years, 57% had a chronic condition, and 41% were admitted to the ICU. Based on chart review, only 41% (n=36) were accurately classified as defined above. 59% (n=51) were inaccurately classified (Table 1), most commonly because they did not have influenza (n=22, 43%). Median LOS was 3 vs 5 days (p=0.01) in accurately vs inaccurately classified patients, respectively. Only one patient had in-hospital ECMO/ death, but this patient had parainfluenza.
Conclusion(s): The majority of patients at our institution classified in administrative data as influenza patients who did not receive oseltamivir were inaccurately classified and these patients had worse outcomes. Although these findings should be replicated at other institutions (multi-center work is ongoing), they illustrate the challenges inherent to comparative effectiveness research using administrative data and should be considered when evaluating prior oseltamivir studies from administrative databases.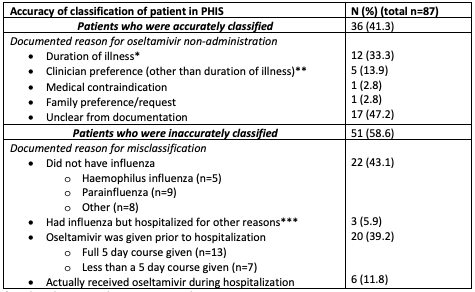
.png)
