Developmental Biology/Cardiac & Pulmonary Development
Developmental Biology/Cardiac & Pulmonary Development 1
180 - Mitochondria dysfunction in cardiac disease of prematurity
Publication Number: 180.403
- GO
Gaston Ofman, MD
University of Oklahoma Health Sciences Center
oklahoma City, Oklahoma, United States
Presenting Author(s)
Background:
Young adults born preterm have reduced cardiac bi-ventricular volume and function contributing to a 17-fold increased risk of heart failure and reduced exercise tolerance. Mitochondria are key for a normal fetal-neonatal cardiac transition, but mitochondria biogenesis does not occur until the last weeks of pregnancy. In addition, hyperoxia is a major contributor of diseases of prematurity. Yet, there is limited knowledge on the effects of this factors in cardiac disease of prematurity.
Objective:
To study the effects of limited cardiac mitochondria pool and hyperoxia exposure on the fetal-neonatal cardiac transition.
Design/Methods:
To assess limited cardiac mitochondrial capacity at birth, we generated mice with cardiomyocyte deletion of Transcription Factor A (TFAM-KO), known to regulate mitochondrial number. Cardiac function and structure were assessed as young adults at P50.
To study the effects of neonatal hyperoxia, Sprague-Dawley pups were exposed to room air (controls) or 80% O2 at postnatal days 1 to 14. Mitochondrial oxygen consumption rate (OCR) and hydrogen peroxide (H2O2) production was assessed in fresh left ventricular fibers at P14 and at P105.
Results:
TFAM-KO mice survive into young adulthood but develop heart failure characterized by reduced ejection fraction and cardiac output (Fig. 1A). TFAM-KO mice also exhibit increased enlarged hearts, resembling cardiac changes seen in preterm born individuals (Fig. 1B). Cardiomyocyte mitosis was found to be increased in TFAM-KO mice (fig. 1C).
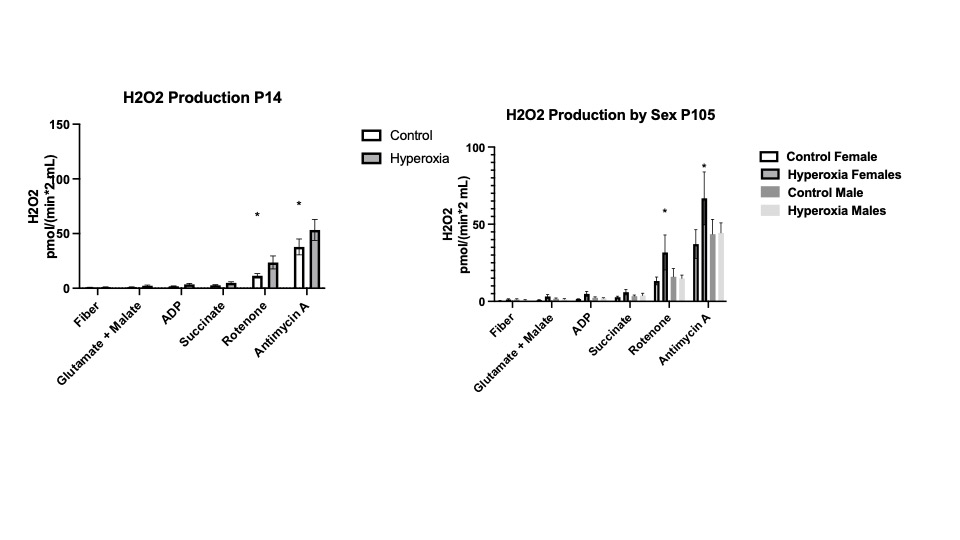
Hyperoxia exposed rats had heavier hearts at P14 that resolved at P105 (Figure 2A). Hyperoxia exposed pups showed an increase OCR compared to control P14 pups that was mainly driven by complex IV (Fig 2B). OCR in P105 female rats remained higher but decreased in males (Fig 2C). Hyperoxia exposed rats generated a significantly increase level of H2O2 than control hearts at P14 that only persisted in females at P105 (Figure 3). Mitochondria integrity, fusion and fission measured by Mitofussin-1, mitofussin-2, OPA1 were not altered between the 2 groups.
Conclusion(s):
When born with limited cardiac mitochondria pool, mice develop cardiac disease similar to those of preterm babies with worsening ventricular function and enlarged hearts. In addition, hyperoxic exposed rats exposed P14 exhibit an increase OCR and elevated production of ROS that persisted in females at P105 with males having less OCR at P105. When completed, this studies will reveal the effects of mitochondria and hyperoxia on the neonatal heart allowing for possible future interventions to prevent heart disease of premature children.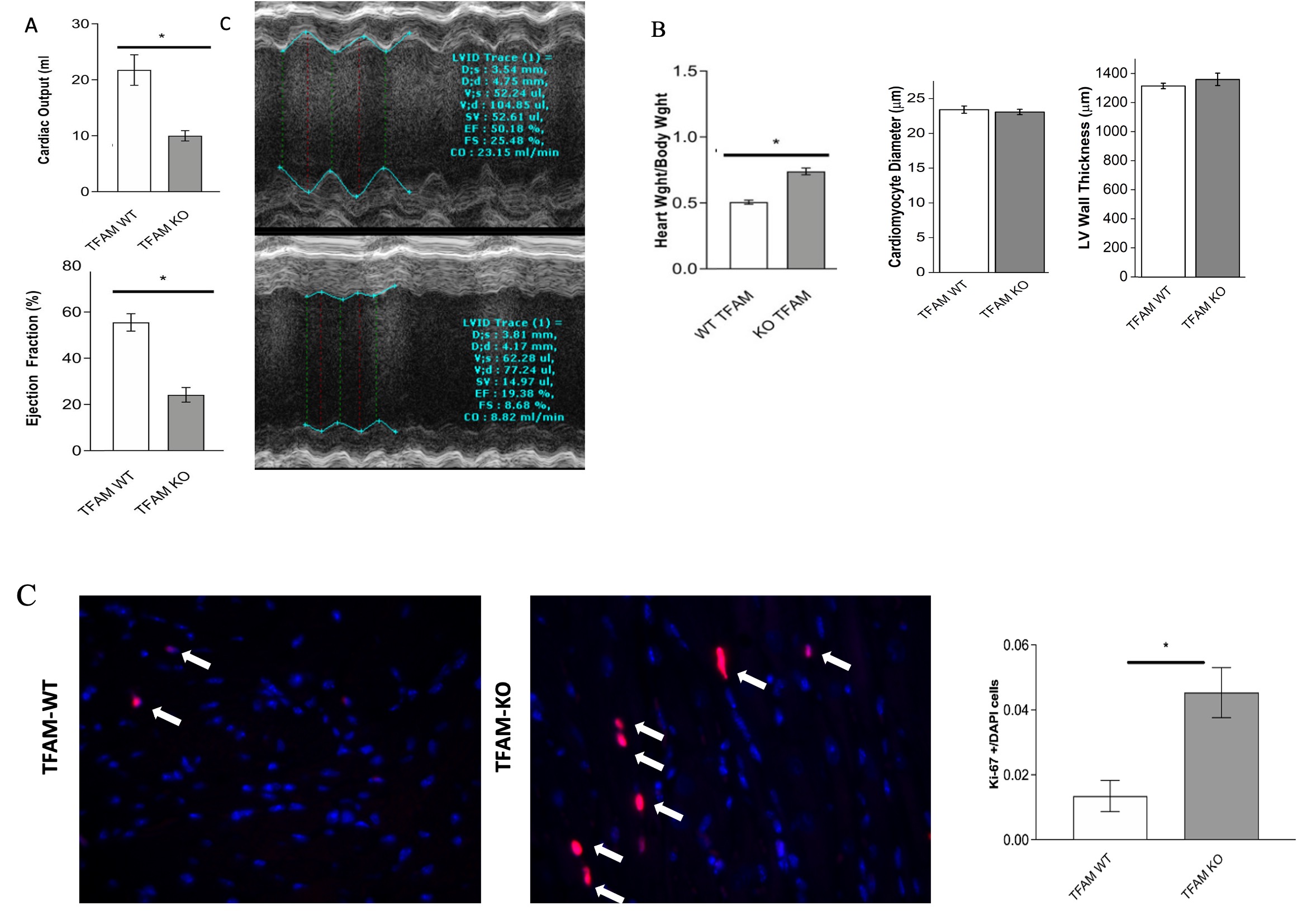
.jpg)